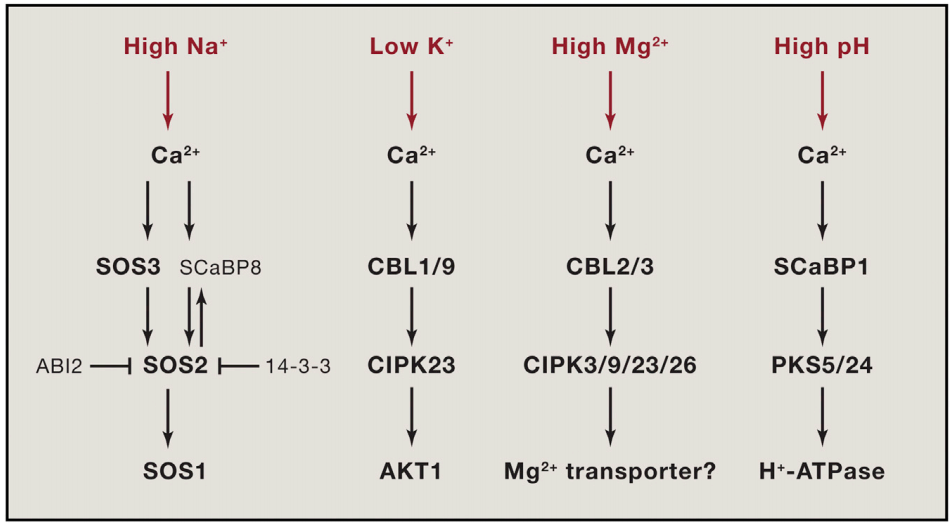
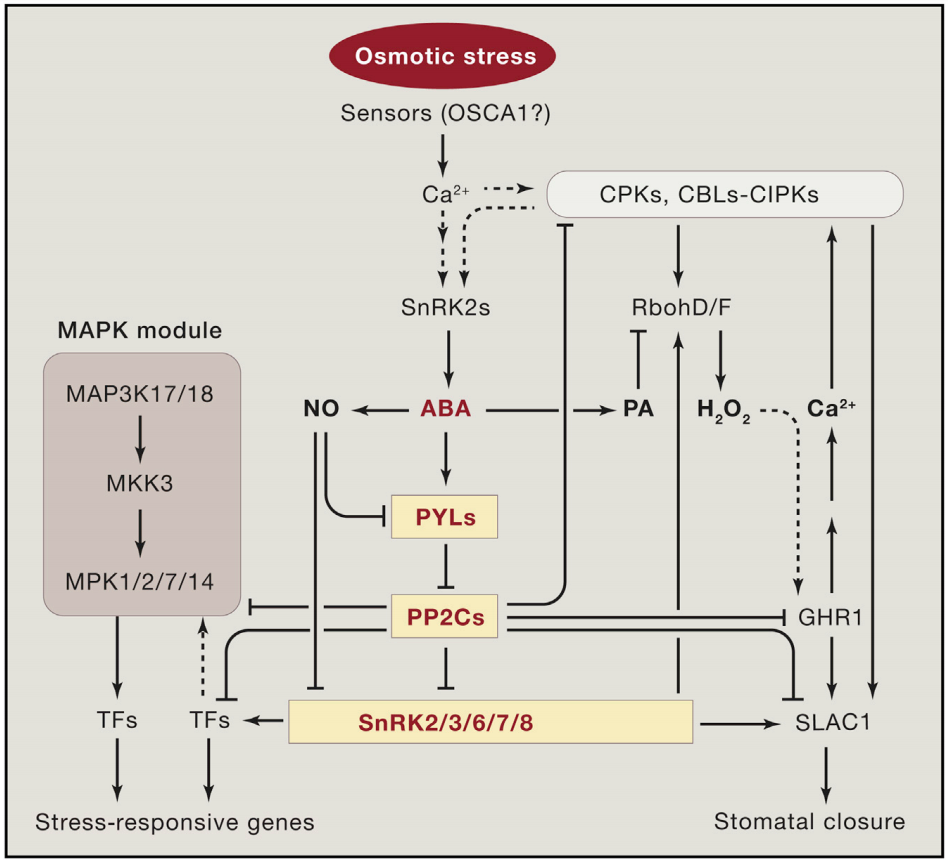
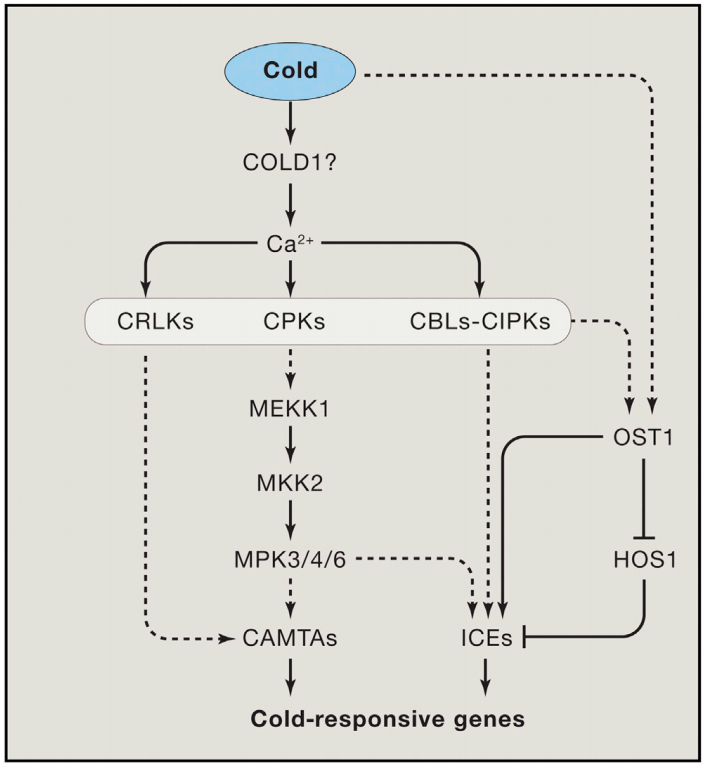
植物Ca2+解码机制已经被广泛研究,包括许多解码器,从钙调素(CaM),一个高度保守的Ca2+传感器,到植物特异性钙调素样(CML)蛋白;钙调磷酸酶B样蛋白(CBLs)及其相互作用的激酶(CIPKs);以及钙依赖蛋白激酶(CDPKs或CPKs)(DeFalco et al., 2010)。相比之下,我们对Ca2+编码过程所知甚少,该过程涉及Ca2+通道和转运体的功能与调节。尽管已经鉴定出几个植物基因家族编码与动物中已知的Ca2+可渗透通道相关的蛋白质,包括那些与环核苷酸门控通道(CNGCs)和谷氨酸受体(GLRs)相似的蛋白质,但这些通道如何在各种信号传导过程中协同或独立地编码特定的Ca2+信号,在很大程度上仍未被探索。本文伯小远将带着大家了解一些这方面的研究进展,与此同时,小远也会为大家简单介绍非生物应激反应中钙信号的编码机制。
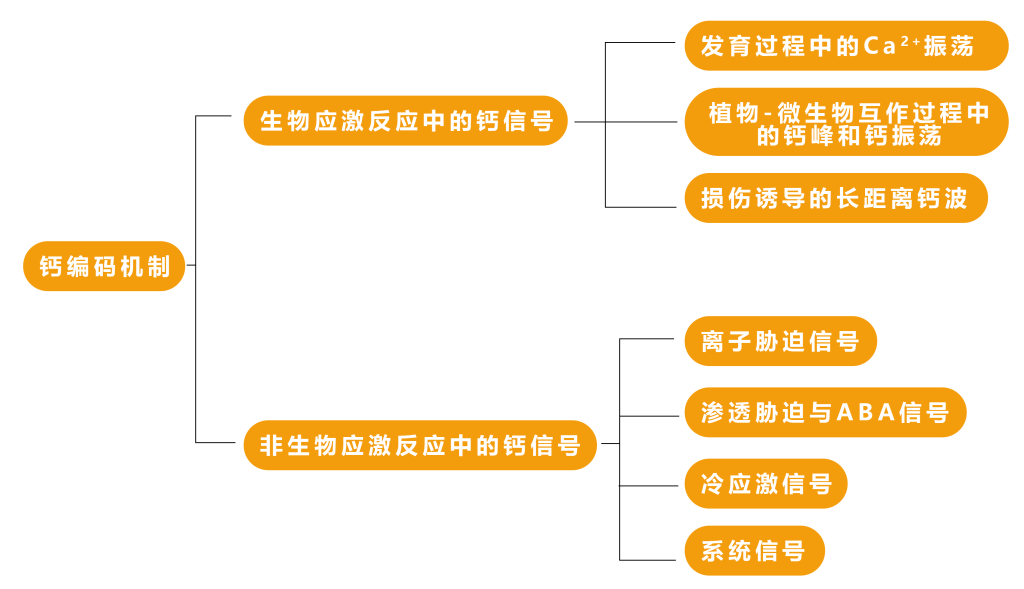
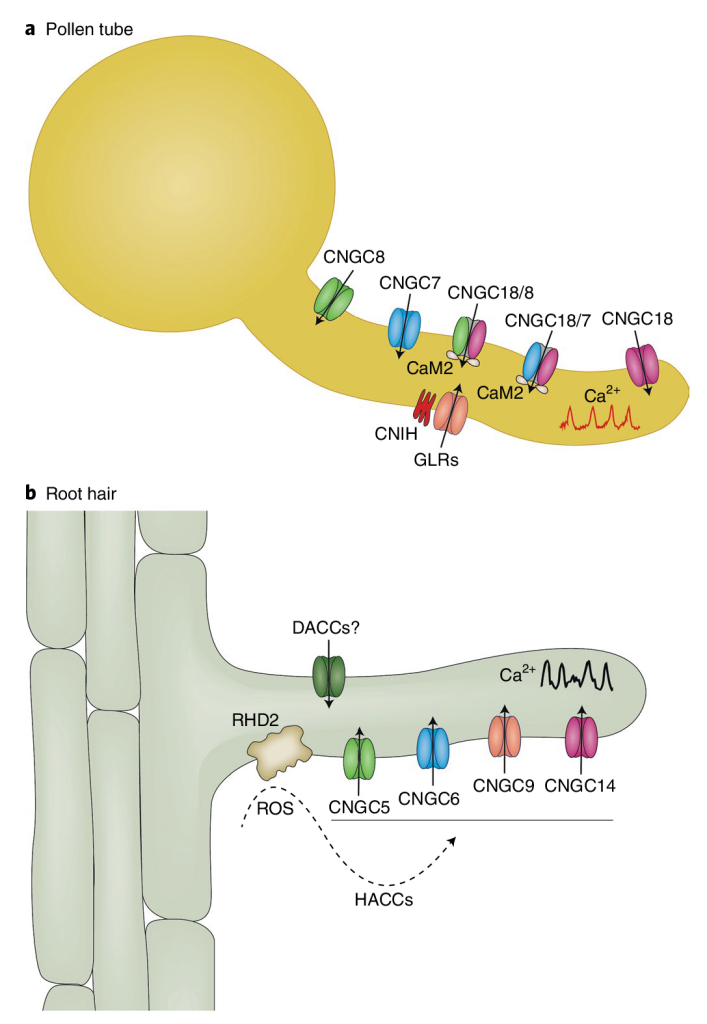
未来的工作应指向探讨CNGCs和GLRs在控制花粉管Ca2+振荡中的功能关系,以及这些通道如何被多肽及其受体等其他信号调控的机制(Johnson et al., 2019)。
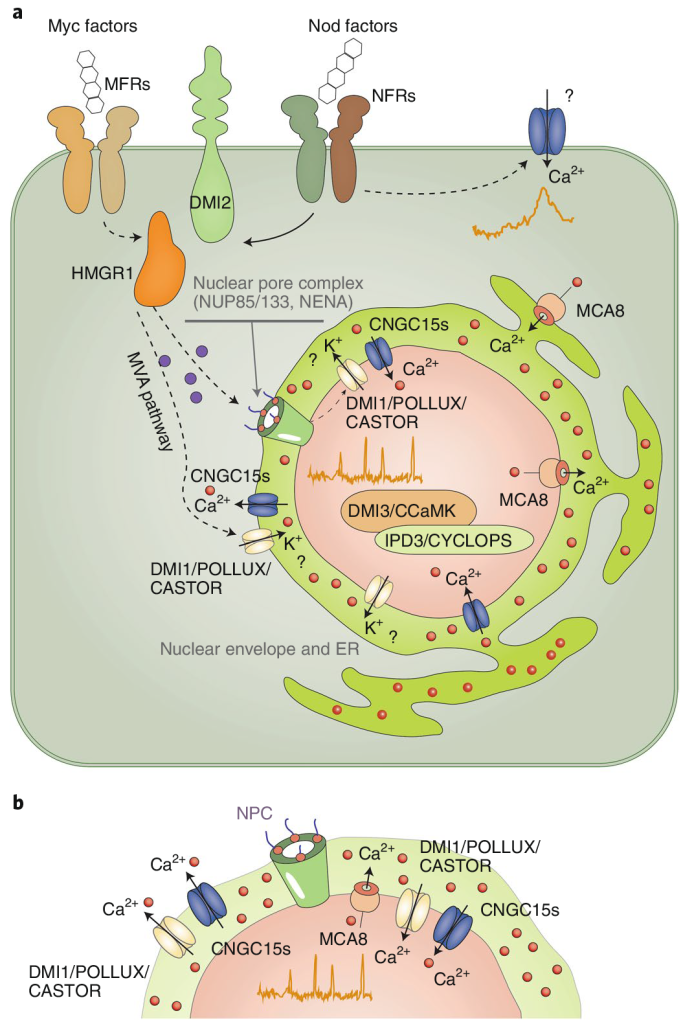
在共生关系建立的起始阶段,植物细胞核内Ca2+浓度会按一定幅度和频率波动(钙振荡)。这种早期的钙信号是驱动共生关系建立所必需的。共生微生物的信号分子(如丛植菌根真菌的菌根因子和根瘤菌的结瘤因子)被宿主细胞表面受体识别后,激活下游信号转导蛋白,进而激活核周内质网定位的通道蛋白和转运蛋白DMI1、CNGC15及MCA8(Ca2+-ATPase),被激活的通道蛋白和转运蛋白组成钙信号“编码器”(图3a)。因内质网腔Ca2+浓度远高于细胞核质,当钙信号编码器激活后,由DMI1和CNGC15将Ca2+快速运到核质空间(钙激增),过高的核质Ca2+立即被MCA8泵回内质网腔,使核质钙离子水平下调(钙衰减),从而为下一次钙离子流入细胞核做准备。Ca2+通过钙“编码器”周期性流进和流出细胞核,从而使细胞核内钙离子浓度产生振荡。振荡的钙信号被细胞核定位的Ca2+/钙调蛋白依赖的蛋白激酶CCaMK(解码器)识别,以磷酸化的方式激活下游转录因子,启动早期共生信号(图3b)。
在模式植物拟南芥中的研究发现,Ca2+波和慢波动电位可以作为长距离信号的载体,驱动植物系统性的信号传递。而这其中细胞膜钙离子通道蛋白GLR3.3,GLR3.6及质子泵AHA1可能参与了植物长距离Ca2+波和慢波动电位的产生和传递。GLRs作为钙离子通道,同时还是谷氨酸受体。当植物被昆虫或其它动物啃食后,维管束内大量的谷氨酸被释放到细胞外,从而激活GLR通道活性使Ca2+进入细胞质内。Ca2+及其他离子的进出引起细胞膜电位变化,形成在器官水平测到的慢波动电位。Ca2+波,慢电位和H2O2协同建立长距离信号,介导非损伤组织的响应(如茉莉酸合成),从而抵御昆虫啃食。
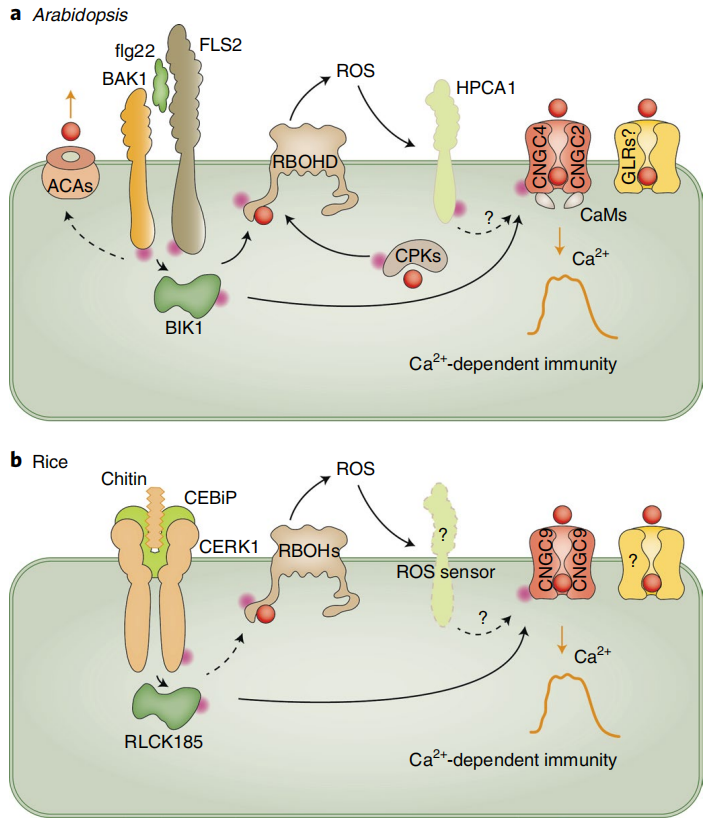
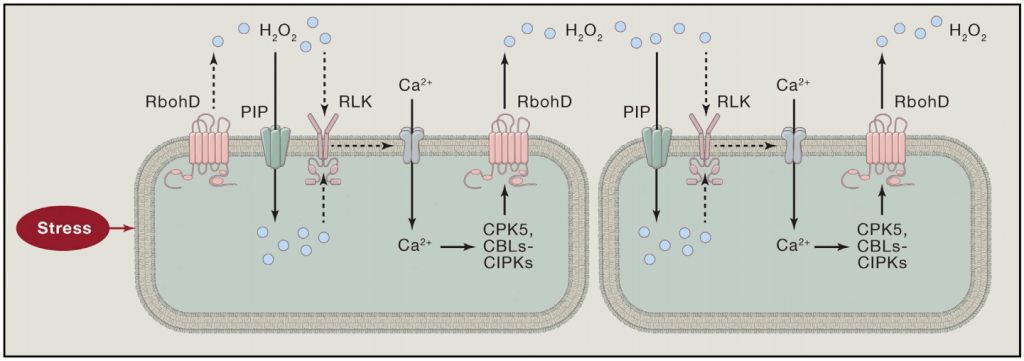
局部暴露于压力下会产生H2O2和Ca2+信号。Ca2+信号可以激活CPKs和CBLs-CIPKs,从而磷酸化并激活RbohD。激活的RbohD产生H2O2,H2O2通过细胞壁扩散到邻近的细胞,在那里它通过像GHR1这样的RLK诱导Ca2+信号。H2O2可以激活细胞表面的Ca2+信号,也可以通过PIP水通道进入细胞,激活细胞内的Ca2+信号。Ca2+和H2O2信号之间的相互激活产生自传播的Ca2+和ROS波,这些波可以传播到远处的组织,引起系统获得性驯化反应。
Brost C, Studtrucker T, Reimann R, et al. Multiple cyclic nucleotide‐gated channels coordinate calcium oscillations and polar growth of root hairs[J]. The Plant Journal, 2019, 99(5): 910-923.
Chinnusamy V, Zhu J, Zhu J K. Cold stress regulation of gene expression in plants[J]. Trends in plant science, 2007, 12(10): 444-451.
Tian W, Wang C, Gao Q, et al. Calcium spikes, waves and oscillations in plant development and biotic interactions[J]. Nature Plants, 2020, 6(7): 750-759.
DeFalco T A, Bender K W, Snedden W A. Breaking the code: Ca2+ sensors in plant signalling[J]. Biochemical Journal, 2010, 425(1): 27-40.
Demidchik V, Bowen H C, Maathuis F J M, et al. Arabidopsis thaliana root non‐selective cation channels mediate calcium uptake and are involved in growth[J]. The Plant Journal, 2002, 32(5): 799-808.
Gao Q F, Gu L L, Wang H Q, et al. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis[J]. Proceedings of the national academy of sciences, 2016, 113(11): 3096-3101.
Granqvist E, Sun J, Op den Camp R, et al. Bacterial‐induced calcium oscillations are common to nitrogen‐fixing associations of nodulating legumes and non‐legumes[J]. New Phytologist, 2015, 207(3): 551-558.
Heckman D S, Geiser D M, Eidell B R, et al. Molecular evidence for the early colonization of land by fungi and plants[J]. Science, 2001, 293(5532): 1129-1133.
Johnson M A, Harper J F, Palanivelu R. A fruitful journey: pollen tube navigation from germination to fertilization[J]. Annual review of plant biology, 2019, 70: 809-837.
Jones J D G, Dangl J L. The plant immune system[J]. Nature, 2006, 444(7117): 323-329.
Kiegle E, Gilliham M, Haseloff J, et al. Hyperpolarisation‐activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots[J]. The Plant Journal, 2000, 21(2): 225-229.
Kistner C, Parniske M. Evolution of signal transduction in intracellular symbiosis[J]. Trends in plant science, 2002, 7(11): 511-518.
Landoni M, De Francesco A, Galbiati M, et al. A loss-of-function mutation in Calmodulin2 gene affects pollen germination in Arabidopsis thaliana[J]. Plant molecular biology, 2010, 74: 235-247.
Michard E, Lima P T, Borges F, et al. Glutamate receptor–like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine[J]. Science, 2011, 332(6028): 434-437.
Miedema H, Demidchik V, Véry A A, et al. Two voltage‐dependent calcium channels co‐exist in the apical plasma membrane of Arabidopsis thaliana root hairs[J]. New Phytologist, 2008, 179(2): 378-385.
Oldroyd G E D. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants[J]. Nature Reviews Microbiology, 2013, 11(4): 252-263.
Pan Y, Chai X, Gao Q, et al. Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities[J]. Developmental cell, 2019, 48(5): 710-725. e5.
Svistoonoff S, Hocher V, Gherbi H. Actinorhizal root nodule symbioses: what is signalling telling on the origins of nodulation?[J]. Current Opinion in Plant Biology, 2014, 20: 11-18.
Tan Y Q, Yang Y, Zhang A, et al. Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels[J]. Plant communications, 2020, 1(1): 100001.
Véry A A, Davies J M. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs[J]. Proceedings of the National Academy of Sciences, 2000, 97(17): 9801-9806.
Wang B, Qiu Y L. Phylogenetic distribution and evolution of mycorrhizas in land plants[J]. Mycorrhiza, 2006, 16: 299-363.
Wudick M M, Portes M T, Michard E, et al. CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis[J]. Science, 2018, 360(6388): 533-536.
Zhang S, Pan Y, Tian W, et al. Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs[J]. Molecular plant, 2017, 10(7): 1004-1006.
Zhu J K. Abiotic stress signaling and responses in plants[J]. Cell, 2016, 167(2): 313-324.
Zipfel C, Oldroyd G E D. Plant signalling in symbiosis and immunity[J]. Nature, 2017, 543(7645): 328-336.