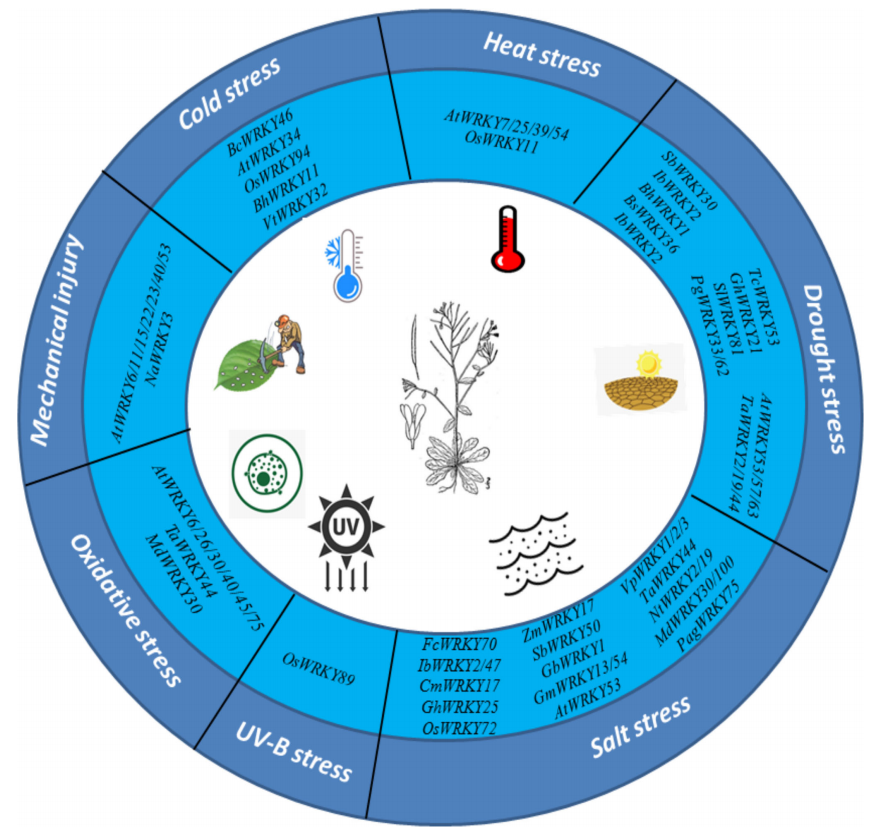
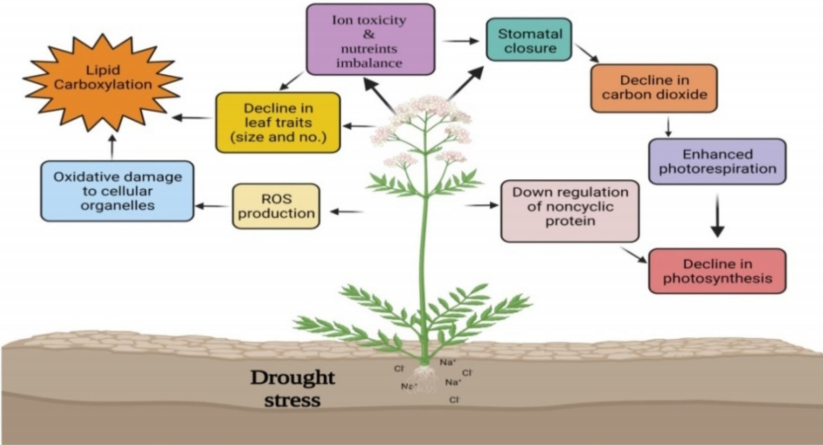
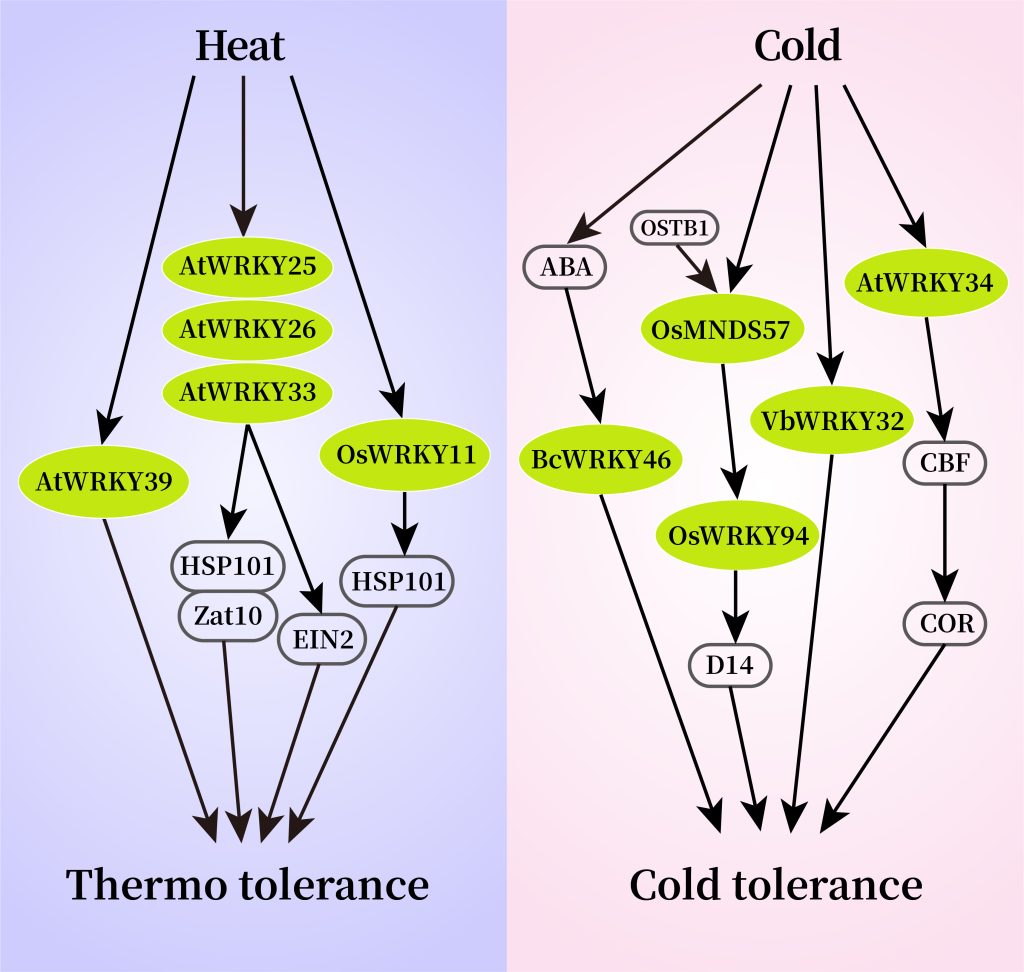
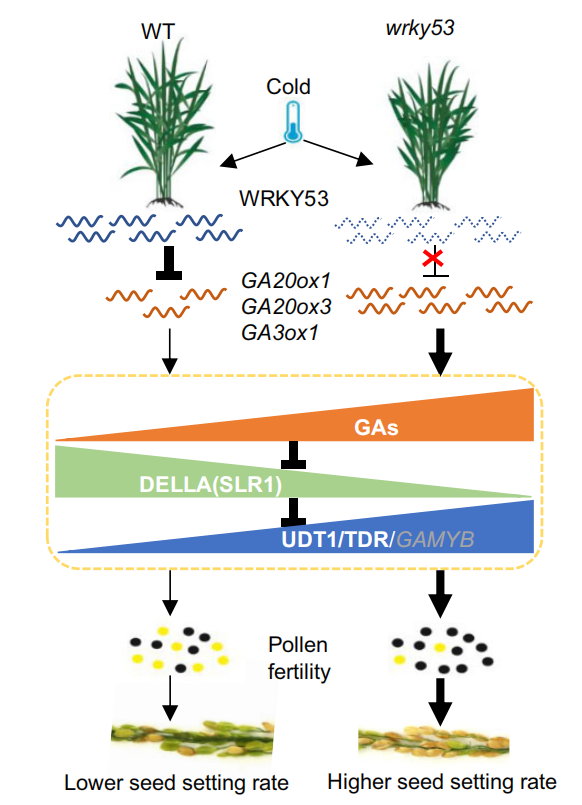
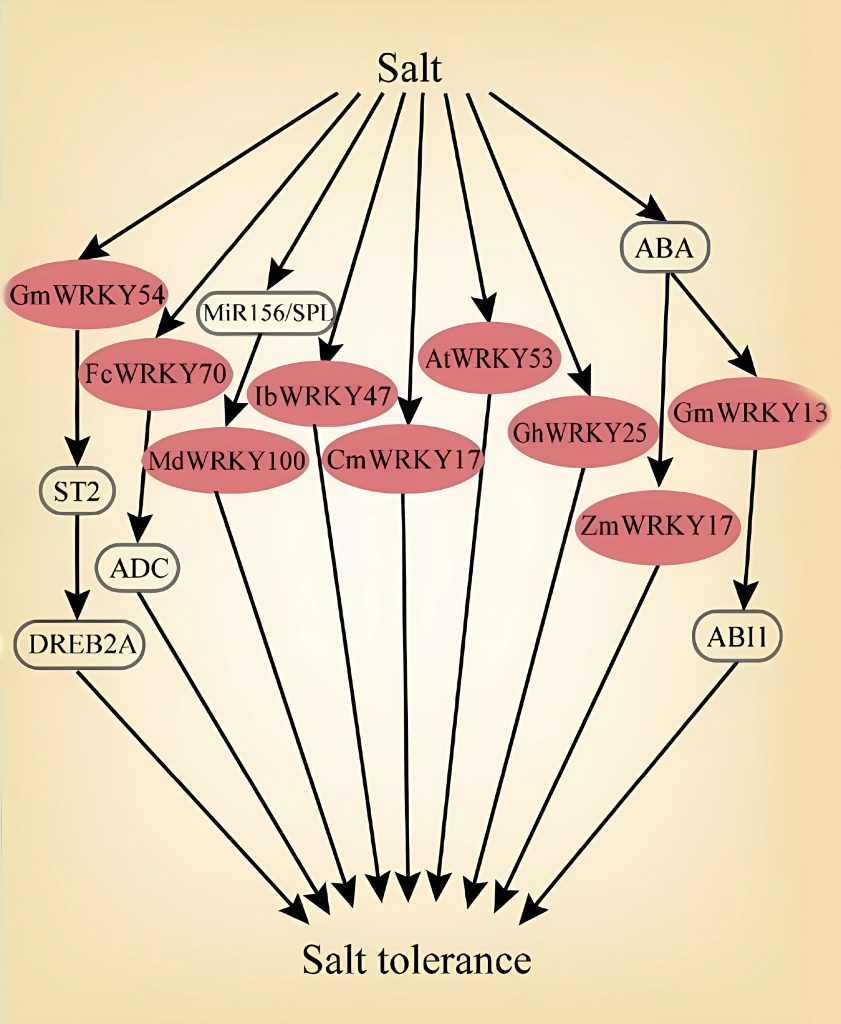
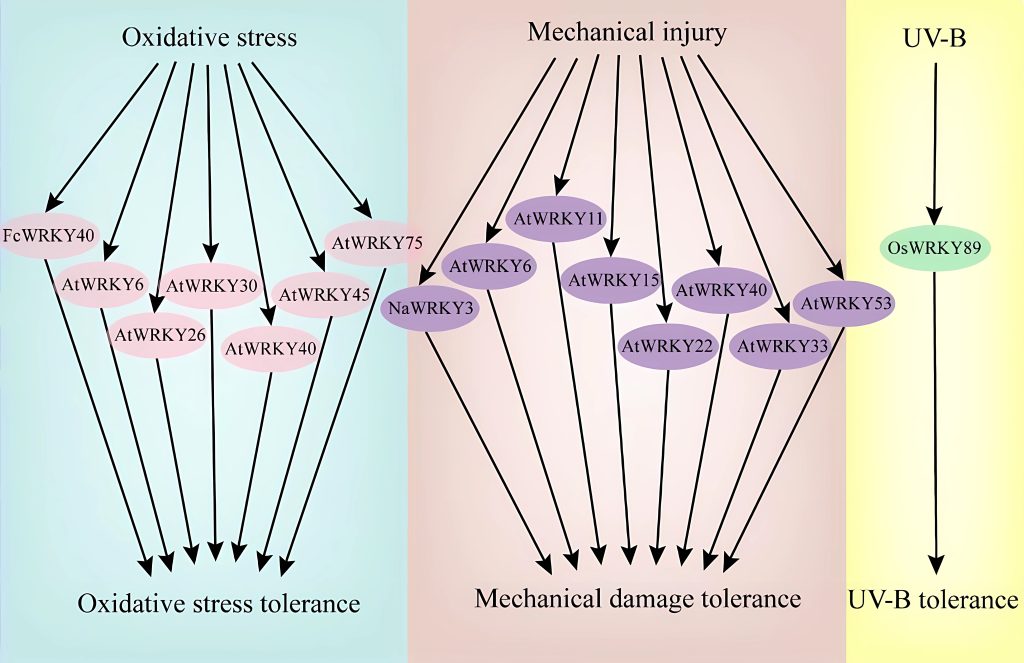
图3 部分WRKY转录因子响应温度胁迫的信号通路图(Li et al., 2020)。
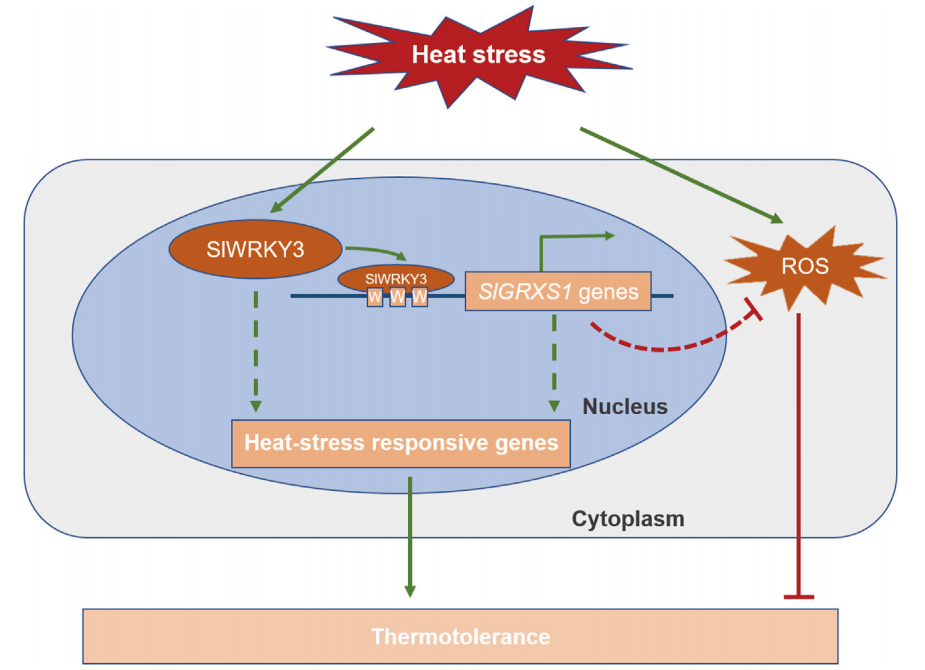
在高温胁迫下,拟南芥WRKY25、WRKY26和WRKY33可以通过调节Hsp101和Zat10基因来提高转基因拟南芥对高温胁迫的耐受性(Li et al., 2011)。遭受高温胁迫的植物可以通过乙烯激活氧化应激反应,进一步研究发现,AtWRKY25、AtWRKY26和AtWRKY33调控高温诱导的乙烯依赖反应过程,并表现出正向交叉调控的作用。由此说明,AtWRKYs正向调节高温应激反应的乙烯激活途径,并在植物的耐热性中发挥协同调控作用。在水稻中,WRKY10过表达水稻植株对高温胁迫的敏感性明显高于对照,而WRKY10功能缺失突变则增强了植株耐热性。进一步研究表明,WRKY10过表达促进了活性氧(ROS)在叶绿体和质外体中的积累,并诱导热激转录因子和蛋白基因的表达(Chen et al., 2022)。在番茄中,WRKY3可以通过直接结合SlGRXS1基因簇的启动子并激活它们的表达和活性氧(ROS)的清除,从而正向调控番茄的耐热性(图4)(Wang et al., 2022)。
图4 SlWRKY3调控SlGRXS1s表达增强番茄耐热性(Wang et al., 2022)。
在低温胁迫下,马鞭草VbWRKY32作为正调节因子,可以通过上调冷反应相关基因的转录水平,增加植株抗氧化活性,维持细胞膜稳定性,增强渗透压调节能力,从而提高植物在冷胁迫下的生存能力(Wang et al., 2020)。在水稻中,OsWRKY53作为负调控因子,可以通过负调控花药GA含量,来影响水稻孕穗期的耐冷性(图5),与野生型相比,OsWRKY53-OE表现为花粉育性差、结实率低,而oswrky53突变体在孕穗期低温胁迫后,相比于野生型植株结实率高、花粉活力强(Tang et al., 2022)。在油菜中,BcWRKY46基因受低温和ABA强烈诱导表达,能够激活ABA信号通路中的相关基因,提高植物的低温耐受性(Wang et al., 2012)。此外,植物还可以在不利环境中通过协调器官发育来响应温度变化。在低温下,水稻MADS-Box转录因子OsMADS57及其相互作用蛋白OsTB1协同激活OsWRKY94的转录调控,通过抑制器官发育基因D14的转录来防止分蘖(Chen et al., 2018)。
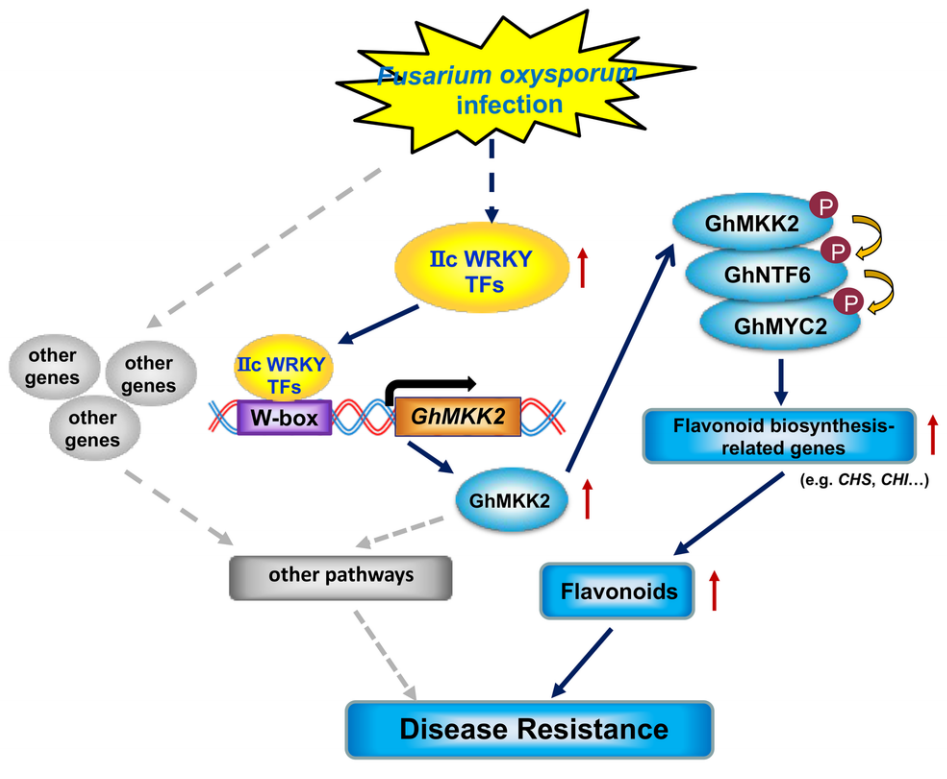
图8 IIc WRKY TF-GhMKK2-GhNTF6-GhMYC2途径对抗Fov侵染的作用模式图(Wang et al., 2022)。
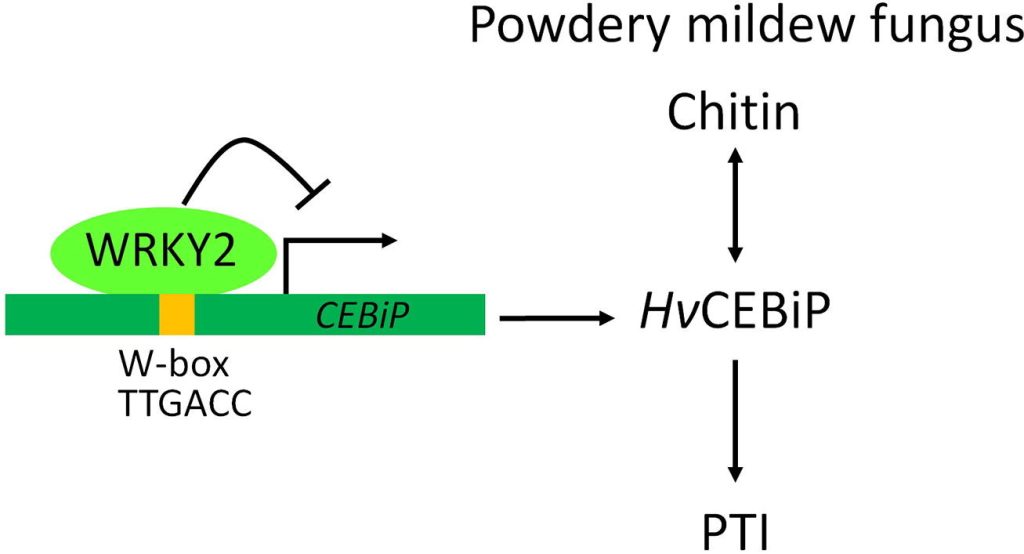
另外,也有些WRKY转录因子在植物对病原菌胁迫的防御中起负向调控作用。TaWRKY3是抗病抑制因子,而SnRK1可以通过磷酸化和破坏TaWRKY3阻遏子的稳定性来提高小麦对白粉病的免疫力(Han et al., 2020)。过量表达OsWRKY76会使得转基因水稻对稻瘟病(Magnaporthe oryzae)的敏感性增强。AtWRKY25在SA介导的紫丁香假单胞菌(Pseudomonas syringae)防御反应中发挥负调控作用。相似的,AtWRKY38或AtWRKY62的过量表达同样能够降低拟南芥对紫丁香假单胞菌的抗性,而突变体对紫丁香假单胞菌的抗性则增强。Yu等人还发现,WRKY2可以通过结合到CEBiP的启动子区调控CEBiP的表达,在病原菌侵染早期阻遏大麦的抗病反应(图9)(Yu et al., 2022)。
References:
Atamian HS, Eulgem T, Kaloshian I. SlWRKY70 is required for Mi-1-mediated resistance to aphids and nematodes in tomato. Planta. 2012, 235(2): 299-309.
An X, Jin G, Luo X, et al. Transcriptome analysis and transcription factors responsive to drought stress in Hibiscus cannabinus. PeerJ. 2020, 8: e8470.
Cheong YH, Chang HS, Gupta R, et al. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002, 129(2): 661-77.
Chen L, Zhao Y, Xu S, et al. OsMADS57 together with OsTB1 coordinates transcription of its target OsWRKY94 and D14 to switch its organogenesis to defense for cold adaptation in rice. New Phytol. 2018, 218(1): 219-231.
Chen S, Cao H, Huang B, et al. The WRKY10-VQ8 module safely and effectively regulates rice thermotolerance. Plant Cell Environ. 2022, 45(7): 2126-2144.
Grunewald W, Karimi M, Wieczorek K, et al. A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiol. 2008, 148(1): 358-68.
Han X, Zhang L, Zhao L, et al. SnRK1 Phosphorylates and Destabilizes WRKY3 to Enhance Barley Immunity to Powdery Mildew. Plant Commun. 2020, 1(4): 100083.
Hou L, Fan X, Hao J, et al. Negative regulation by transcription factor VvWRKY13 in drought stress of Vitis vinifera L. Plant Physiol Biochem. 2020, 148: 114-121.
Jiang, Y.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2008, 69, 91-105.
Jiang Y, Deyholos MK. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol. 2009, 69(1-2): 91-105.
Kilian J, Whitehead D, Horak J, et al. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50(2): 347-63.
Kloth KJ, Wiegers GL, Busscher-Lange J, et al. AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J Exp Bot. 2016, 67(11): 3383-96.
Khoso MA, Hussain A, Ritonga FN, et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front Plant Sci. 2022, 13: 1039329.
Li S, Fu Q, Chen L, et al. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta. 2011, 233(6): 1237-52.
Li W, Pang S, Lu Z, et al. Function and Mechanism of WRKY Transcription Factors in Abiotic Stress Responses of Plants. Plants (Basel). 2020, 9(11): 1515.
Liu X, Wang Y, Zhu H, et al. Natural allelic variation confers high resistance to sweet potato weevils in sweet potato. Nat Plants. 2022, (11): 1233-1244.
Ma Y, Xue H, Zhang F, et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol J. 2021, 19(2): 311-323.
Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001, 28(2): 123-33.
Ren X, Chen Z, Liu Y, et al. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010, 63(3): 417-29.
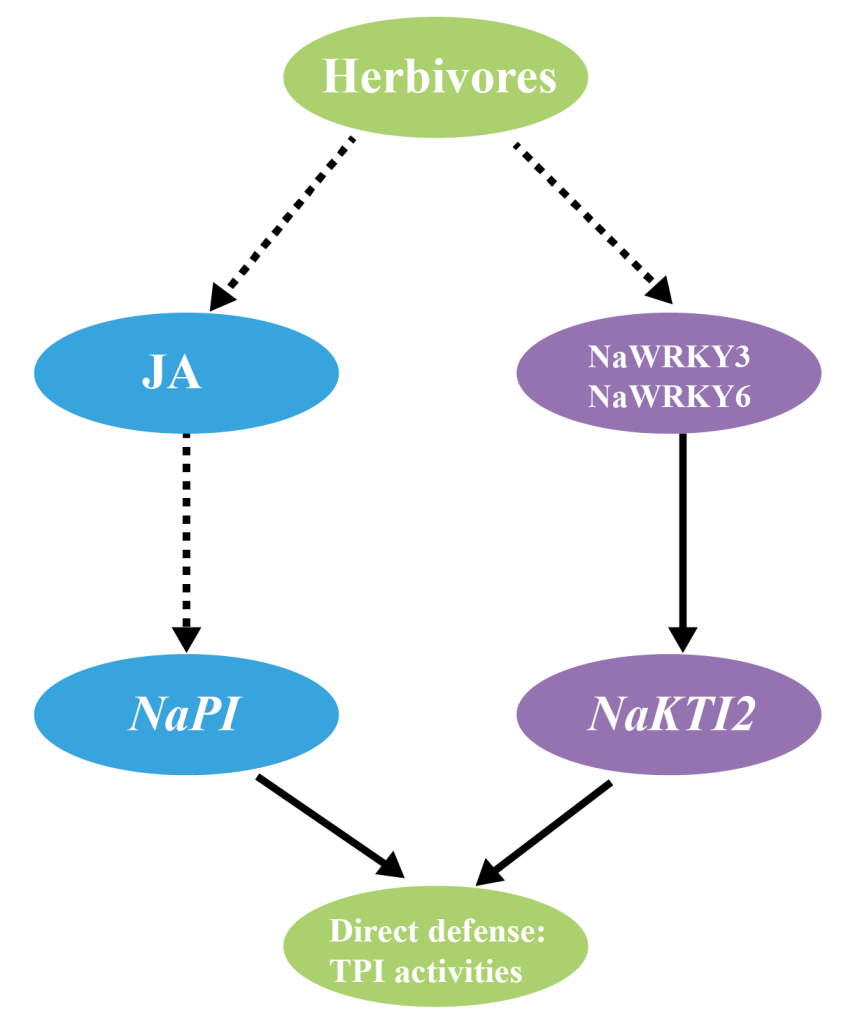
Skibbe M, Qu N, Galis I, et al. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell. 2008, 20(7): 1984-2000.
Shen H, Liu C, Zhang Y, et al. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol Biol. 2012, 80(3): 241-53.
Tang J, Tian X, Mei E, et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell. 2022, 34(11): 4495-4515.
Wang H, Hao J, Chen X, et al. Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol Biol. 2007, 65(6): 799-815.
Wang F, Hou X, Tang J, et al. A novel cold-inducible gene from Pak-choi (Brassica campestris ssp. chinensis), BcWRKY46, enhances the cold, salt and dehydration stress tolerance in transgenic tobacco. Mol Biol Rep. 2012, 39(4): 4553-64.
Wang CT, Ru JN, Liu YW, et al. The Maize WRKY Transcription Factor ZmWRKY40 Confers Drought Resistance in Transgenic Arabidopsis. Int J Mol Sci. 2018, 19(9): 2580.
Wang Y, Gai WX, Yuan LD, et al. Heat-inducible SlWRKY3 confers thermotolerance by activating the SlGRXS1 gene cluster in tomato. Horticultural Plant Journal, 2022.
Wang MQ, Huang QX, Lin P, et al. The Overexpression of a Transcription Factor Gene VbWRKY32 Enhances the Cold Tolerance in Verbena bonariensis. Front Plant Sci. 2020, 10: 1746.
Wang L, Guo D, Zhao G, et al. Group IIc WRKY transcription factors regulate cotton resistance to Fusarium oxysporum by promoting GhMKK2-mediated flavonoid biosynthesis. New Phytol. 2022, 236(1): 249-265.
Xiao S, Ming Y, Hu Q, et al. GhWRKY41 forms a positive feedback regulation loop and increases cotton defence response against Verticillium dahliae by regulating phenylpropanoid metabolism. Plant Biotechnol J. 2023.
Yin M, Song N, Chen S, Wu J. NaKTI2, a Kunitz trypsin inhibitor transcriptionally regulated by NaWRKY3 and NaWRKY6, is required for herbivore resistance in Nicotiana attenuata. Plant Cell Rep. 2021, 40(1): 97-109.
Yu DS, Fan RC, Zhang L, et al. HvWRKY2 acts as an immunity suppressor and targets HvCEBiP to regulate powdery mildew resistance in barley. crop journal, 2022.
Zhou QY, Tian AG, Zou HF, et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J. 2008, 6(5): 486-503.
Zhou QY, Tian AG, Zou HF, et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J. 2008, 6(5): 486-503.